Fuels

Utilization of CO2 from air or biobased resources for the production of fuels
Nowadays, the production of fuels from CO2 and H2 via Power-to-Liquid (PtL) technologies becomes more and more important. In general, these PtL pathways are an excellent opportunity to lower CO2 emissions or utilize CO2 and transform it into valuable and sustainable fuels. Additionally, for an effective reduction of the carbon footprint, the H2 is produced from renewable sources, such as wind and solar-powered water electrolysis, or fossil resources combined with carbon capture and storage technology.
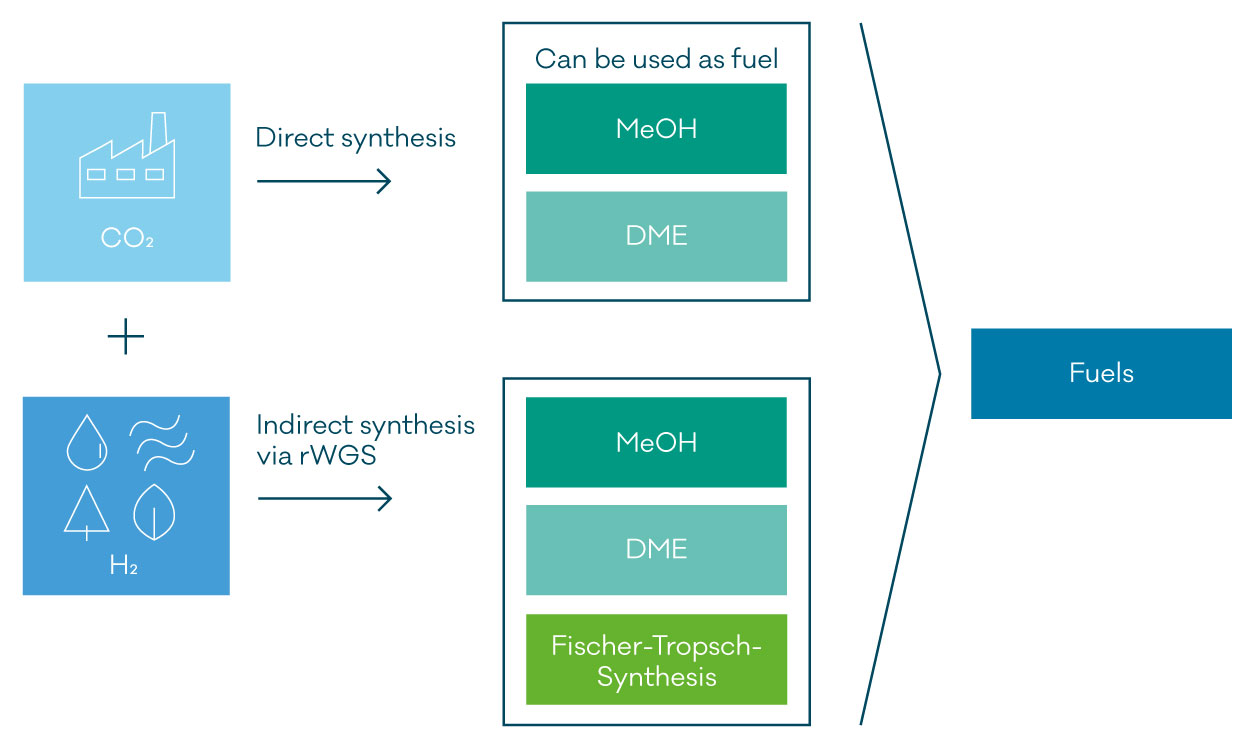
The most promising routes include those with methanol, DME as C1 building block, and Fischer-Tropsch synthesis. MeOH and DME can either be used directly as fuels or be converted into gasoline via different technologies. FT products can be upgraded via hydrocracking and isomerization to obtain fuels. With regards to FTS, this known technology produces different types of fuel such as gasoline, kerosene, or diesel and therefore offers a higher degree of flexibility. To apply conventional FT or MeOH-synthesis, CO2 and H2 are converted into synthesis gas via rWGS reaction first. Latest R&D activities address the direct MeOH or FT synthesis from CO2 and H2.
What are the challenges in producing fuels from CO2?
In order to produce fuels directly from CO2, more research on catalyst and process development via laboratory and pilot plant testing is required. In the case of direct MeOH synthesis from CO2, increased water formation and lower equilibrium conversion are observed. Hence new catalysts and reactor designs are needed.
There are different routes available to produce DME which involve the direct conversion of CO2, involves an rWGS step, or lead via MeOH. Here, laboratory tests and pilot plants are needed for upscaling and improved performance of industrial units. MeOH can also be further processed towards oxymethylene ethers, serving as alternative diesel fuel for soot-free combustion.
The most flexible process in terms of the product variety, i.e. the Fischer-Tropsch process uses different metals (catalysts). Not all of them can utilize CO2 directly. Only iron catalysts catalyze the WGS reaction while at the same time yield liquid products with higher selectivity. However, more research, e.g. to improve the conversion rates, needs to be done. In general, additional techno-economic analyses and parameter screening with regards to new reactor concepts or two-stage processes including the rWGS technology are necessary. This is even more valid for smaller and flexible instead of large-scale plants.
How can hte support your research in fuels from CO2?
We support our customers in catalyst and process development as well as technical service including competitive catalyst testing, process optimization, and quality control. Deactivation studies of the catalyst and testing under industrially relevant conditions can be carried out.
Further, we can provide you our extensive expertise in synthesis gas conversion chemistry (Fischer-Tropsch, methanol, DME, higher alcohols) to help you finding tailor-made solutions.
Scientific literature overview of academic and industrial applications
V. Dieterich, A. Buttler, A. Hanel, H. Spliethoff, S. Fendt,
„Power-to-liquid via synthesis of methanol, DME or Fischer-Tropsch-fuels: a review”
Energy & Environmental Science Royal Society of Chemistry, 2020, 3207-3252. DOI.
C. Schulz, P. Kolb, D. Krupp, L. Ritter, A. Haas, M. Soorholtz, T.E. Maldonado, T.B. Thiede, C. Knobloch,
“Preparation and High-Throughput Testing of TiO2-Supported Co Catalysts for Fischer-Tropsch Synthesis“
Catalysts, 2021, 11, (3), 352. DOI.
D. Wang, Z. Xie, M.D. Porosoff, J.G. Chen,
“Recent advances in carbon dioxide hydrogenation to produce olefins and aromatics”
Chem. 2021, 7, 1-35. DOI.
