Methanol Synthesis

Methanol synthesis from CO2
The synthesis of Methanol (MeOH) from CO2 as a major or sole feedstock is discussed as one option to overcome climate changes.
Methanol has one of the highest production capacities among organic base chemicals and is thus of extreme importance for the global chemical industry. One unique feature of MeOH is its versatility. It can be starting material for other oxygen-containing base chemicals (formaldehyde, acetic acid), be used to produce light olefins and fuels via the various methanol-to-hydrocarbons processes and serve as a drop-in fuel in combustions engines.
Methanol as a versatile base chemical
Methanol is available via various large-scale processes starting from synthesis gas. Though the formation from synthesis gas is frequently described by eq. 1, it is well established that the MeOH formation proceeds by hydrogenation of CO2 (eq. 2), and that the CO consuming step is the water gas shift reaction (eq. 3). The synthesis of MeOH from CO2 alone is discussed as one option to overcome the climate changes associated with increasing levels of CO2 emissions and CO2 enrichment in the atmosphere.
2 H2 + CO → CH3OH ΔHR = -91 kJ/mol [Eq. 1]
3 H2 + CO2 → CH3OH + H2O ΔHR = -50 kJ/mol [Eq. 2]
H2O + CO → H2 + CO2 ΔHR = -41 kJ/mol [Eq. 3]
Challenges from using C02 as a Feedstock
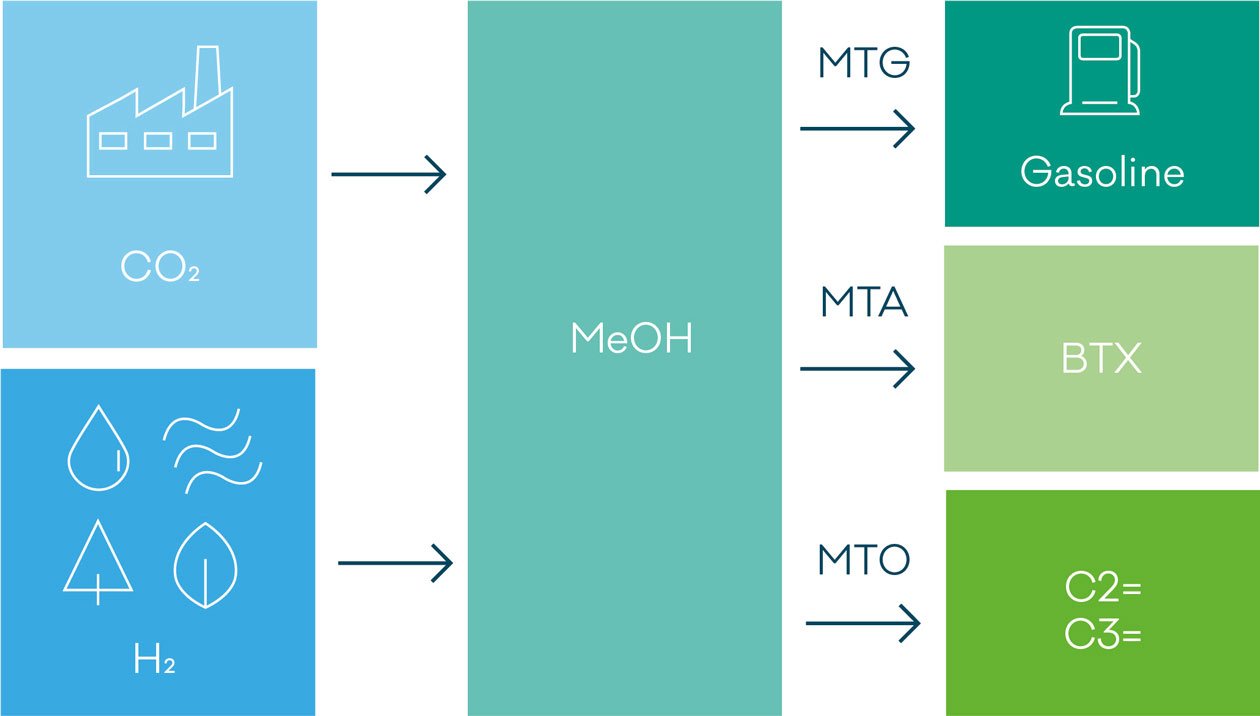
The use of CO2 as major or sole feedstock (see also figure 1) for MeOH synthesis poses two major challenges:
- The reaction rate of the established Cu based catalysts with pure CO2 are lower than with CO rich feed gases
- The selectivity for MeOH can be lower due to formation of CO by the competing reverse water gas shift reaction (equation 3 backward)
How can hte help to overcome the challenges in Methanol synthesis?
hte offers various services in the field of MeOH synthesis from conventional and unconventional feedstocks (i. e. CO2 rich):
- Preparation and testing of novel material compositions, for example with the goal of improving the reaction rate and selectivity with unconventional feedstocks
- Benchmarking of commercial catalysts with regards to activity, selectivity, and stability including protocols for accelerated thermal aging
- Process optimization under conditions that mimic commercial plants, i.e. with separation of the produced MeOH containing liquid inside the reactor system for further analysis and recycling the gaseous fraction of the effluent back to the reactor inlet
LEARN MORE ABOUT METHANOL SYNTHESIS FROM CO2
P. Kolb, T. Kaltschmitt,
"Methanol Synthesis in a Fixed Bed Recycle-reactor System: Effect of once-Through and Recycle Operation on Activity and Productivity"
DGMK Conference Paper, 2017
P. Kolb, T. Kaltschmitt
"Methanol Synthesis: Effects in once-Through and Recycle Operation"
DGMK Conference Presentation, 2017
