Dialkyl Carbonates

Dialkyl Carbonates: Green chemicals and solvents with potential for sustainable synthesis routes
Dialkyl carbonates are considered as green chemicals due to their biodegradability and low toxicity. In addition, they can benefit from sustainable synthesis routes by utilizing CO2 and renewable alcohols (e.g. synthesis of green methanol).
Dialkyl carbonates act as excellent reagents and solvents in chemical applications such as pharmaceuticals, fine chemicals and pesticides. In particular, dimethyl carbonate (DMC) is used as carbonylation and alkylation agent in organic synthesis. Dialkyl carbonate mixtures are used as co-solvent in electrolytes for lithium ion batteries. They are used as solvents in adhesives, coatings, paints and ink formulations. DMC is used in polycarbonate production. Diethyl carbonate (DEC) and DMC are discussed as fuel additives (e.g. as particulate reducing agent for emission control). The demand for dialkyl carbonates is expected to grow further over the coming years. For instance, the DMC demand has doubled (from 396 kton to 786 kton) from 2010 to 2018 with an average annual growth rate of 7.8% according to Nexant.
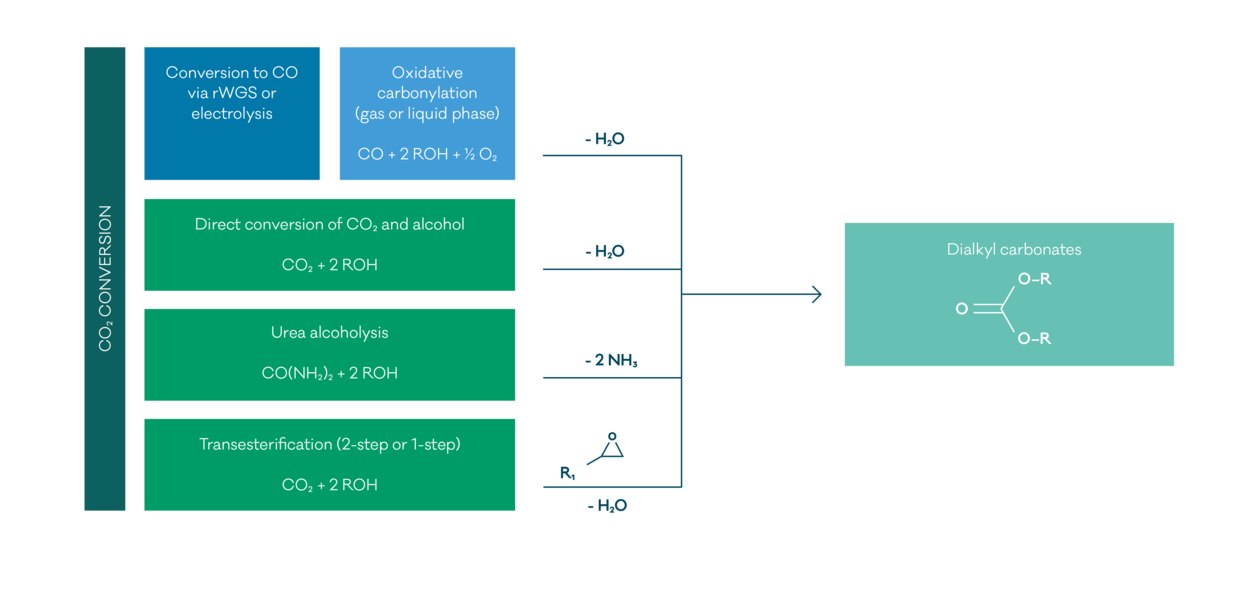
In early days, symmetrical dialkyl carbonates were produced by phosgene alcoholysis. This method was finally banned due to safety and environmental reasons. Alternative ways are oxidative carbonylation of the respective alcohols with carbon monoxide in gas or liquid phase, or the conversion of CO2:
- Direct conversion of CO2 and alcohols
- Alcoholysis of urea
- Transesterification of cyclic carbonates obtained from CO2 and epoxides
What are the challenges in synthesis of dialkyl carbonates?
Direct synthesis from CO2 and alcohols would be the ideal and most sustainable production route. However, this route suffers from the low reactivity of CO2 and thermodynamic limitations and has not left the lab phase so far.
Oxidative carbonylation and the 2-step transesterification process (with an intermediate cyclic carbonate) are the current industrial processes. The development and optimization of active and stable catalysts without major separation efforts is of high importance for these commercial processes.
With the trend towards larger electrolyzers, oxidative carbonylation could profit from renewable energy and unused industrial CO2 sources. In this way, CO can be considered as the electrochemically activated form of CO2. In addition, CO can be obtained from CO2 by reverse water gas shift.
Converting CO2 into urea is another way of capturing and activating CO2. Ammonia is not consumed in this process and can be recycled via the urea production (ideally based on renewable energy sources). Active and stable catalysts for the substitution of the second amine group as well as removal of excess ammonia are key factors.
In the transesterification process the epoxide acts as an activator for CO2. The challenge in the 2-step transesterification is to improve the conversion of the cyclic carbonates as well as the selectivity towards the dialkyl carbonates by avoiding unwanted alcoholysis. Transforming the transesterification process into a 1-step process would reduce investment, production costs and energy demand.
How can hte support your R&D efforts in the area of Dialkyl Carbonates?
hte offers various lab services in process optimization and development of new catalytic processes:
- Fundamental thermodynamic and kinetic studies
- Proof of concept studies for new process developments in batch, CSTR, fixed or fluidized bed reactor
- Screening, optimization and up-scaling of new catalyst formulations with respect to catalyst activity, selectivity and stability
- Finding the optimal reactor system – from batch to continuous operation
- Optimizing process conditions, e.g. operation in gas or liquid phase with the optimal process window (temperature, pressure, flow rate, concentration)
- Investigating catalyst lifetime as well as root-cause of deactivation under industrially relevant process conditions and feedstocks
